During the Second World War, a team of gifted American scientists worked together to create an atomic bomb, which had a power hard to grasp by the human mind. The physics behind this device was discovered when Albert Einstein undertook abstract calculations on the fundamental structure of space and time, known as his theory of special relativity. What at first seemed a purely theoretical endeavor with no practical application soon turned into an almost demonic force—the likes of which the world had never seen. The bomb was used to stop Hitler’s ally Japan, thereby ending the Second World War.
The theory of special relativity
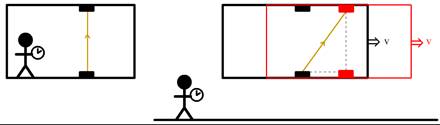
In 1905, Albert Einstein (1879–1955) developed his famous theory of special relativity, which was an abstract mathematical work of theoretical physics on the structure of space and time. The work was based on some curious measurements of the speed of light. It turned out that the speed of light always gave the same value, even when either the observer or the light source was moving. This was a remarkable find for the following reason. Imagine a spaceship moving to the right with a constant velocity. Inside the ship a laser shoots a light ray upwards into a detector. For a person standing inside the ship, the light ray moves straight up (see the left side of Fig. 625). For a person standing on the Earth looking at the ship flying by, however, the light beam moves diagonally as this person also sees the ship moving to the right (see the right side of Fig. 625). Notice the distance traveled by the light is different for both observers! In Newtonian physics this isn’t a problem, since here the light on the right side of the figure gains some extra velocity because of the movement of the ship, and as a result still reaches the detector at the same time. But in the Einsteinian case, light moves just as fast for all observers! This means that light takes longer to reach the detector when seen from Earth! The only way Einstein was able to make sense of this was to assume that time is moving slower if objects move relative to us! And this has to be taken literally. In principle, a person moving with close to the speed of light for, say, a year, might come back to find the Earth hundreds of years older. At this point, we do not have spacecraft that travel fast enough to make this happen to any significant extent, but small subatomic particles in the lab can easily reach these speeds, allowing us to see these effects firsthand.
Fig. 625 – On the left, we see a person in a spaceship measuring how long it takes for light to move vertically. On the right we see a person measuring the same light beam, but now the person is standing on the Earth and sees the ship move by. As a result, the light beam seems to move diagonally, covering a longer distance (S. P. Dinkgreve, worldhistorybook.com)
At the time, the special theory of relativity only seemed to have intellectual significance, but not long after, Einstein showed that his new ideas about space and time also required us to revise our understanding of matter and energy itself. He reasoned that by fusing two atoms together, the resulting atom should have a mass slightly higher than the two original atoms. The energy that was needed to fuse these atoms together had converted into an extra amount of mass, according to his famous equation:
E = mc2
The opposite is also true. When two atoms break up, some mass converts back into energy. The energy released by one atom is incredibly small, but this didn’t stop a small group of people from fantasizing about its applications. One of them was the science-fiction writer H. G. Wells (1866–1946). In his book The World Set Free, he wrote about a bomb so powerful “a man could carry about in a handbag an amount of latent energy sufficient to wreck half a city.” In the story, the bombs were made of “lumps of pure carolinum,” a fictional radio-active element that produced “tremendous pillars of fire,” filling the sky with “flickering lightnings and rushing clouds.” [409] Wells also spoke of buildings being leveled on an unprecedented scale and enormous clouds rising over the city.
Fig. 626 – Albert Einstein (Library of Congress, United States)
Nuclear physics
The first real-life evidence for the conversion of matter into energy came from nuclear physics—the study of atomic nuclei. In 1919, Ernest Rutherford (1871–1937) shot helium nuclei at nitrogen atoms. To his surprise, this caused the nitrogen to absorb part of the helium, turning it into oxygen, finally fulfilling the alchemical goal of transmuting one element into another. This process is called nuclear fusion. In 1932, two students working under his direction shot protons at lithium atoms, causing the lithium nuclei to split into two helium nuclei, which is an example of nuclear fission.
With these discoveries, the Hungarian physicist Leo Szilard (1898–1964) began to believe that an atomic bomb might soon become a reality. He even alerted the British and American governments to inform them about this possibility. Most scientists, however, did not see how Einstein’s theories could be put into practice anytime soon. Even Rutherford called such expectations “moonshine.” [409]
In 1934, the Italian physicist Enrico Fermi (1901–1954) started shooting neutrons at atoms. The neutron, being a neutral particle, could hit the nucleus of an atom without being repelled by its positive charge. As a result, it could hit the nucleus with a much smaller velocity. Since slower particles stay longer in the vicinity of the nucleus, they were more likely to collide. To slow neutrons down, Fermi shot them through various materials, which he called moderators.
In Germany, Otto Hahn (1879–1968), Lise Meitner (1878–1968), and Fritz Strassmann (1902–1980) discovered that a uranium atom would break into two parts of about equal size when hit by a neutron. Calculations by Lise Meitner and Otto Frisch (1904–1979) revealed that the parts produced by the collision weighed less than the original nucleus and that this mass had been converted into energy, exactly according to Einstein’s formula. This was the first observation of mass-energy conversion.
Fig. 627 – Hahn and Meitner at work (c. 1913) (Smithsonian Institution, United States)
Hahn and Strassmann then discovered that the fission of uranium caused several neutrons to “boil off” from the nuclei, which could, in turn, cause other uranium nuclei to fission, creating a chain reaction.
In 1939, alarmed by these developments, Szilard wrote a letter to President Franklin Roosevelt (1882–1945) and convinced Einstein to sign it. We read:
Some recent work by E. Fermi and L. Szilard, which has been communicated to me in manuscript, leads me to expect that the element uranium may be turned into a new and important source of energy in the immediate future. Certain aspects of the situation which have arisen seem to call for watchfulness and if necessary, quick action on the part of the Administration. I believe therefore that it is my duty to bring to your attention the following facts and recommendations.
In the course of the last four months it has been made probable […] that it may be possible to set up a nuclear chain reaction in a large mass of uranium, by which vast amounts of power and large quantities of new radium-like elements would be generated. Now it appears almost certain that this could be achieved in the immediate future.
This new phenomenon would also lead to the construction of bombs, and it is conceivable—though much less certain—that extremely powerful bombs of this type may thus be constructed. A single bomb of this type, carried by boat and exploded in a port, might very well destroy the whole port together with some of the surrounding territory. However, such bombs might very well prove too heavy for transportation by air. [410]
When the president received the letter, Hitler had already invaded Poland. Knowing that the Germans were also actively researching this new source of energy, Roosevelt agreed to fund further research to prevent the Germans from getting hold of such a weapon first.
In 1940, it was discovered that the isotope uranium-235 (U-235) could fission with a neutron, but not the much more abundant uranium-238 (U-238). To create a self-sufficient chain reaction, it was therefore essential to remove the U-238 from the material. At the time, however, there was no effective method to separate the isotopes, as U-235 and U-238 are chemically identical and their masses only differ by less than 1%.
In 1941, the element neptunium was identified. This was the first man-made element that did not occur in nature. Not long after, Glenn Seaborg (1912–1999) discovered that one isotope of neptunium decayed into an element that he later named plutonium. Plutonium-239 turned out to be 1.7 times more likely to fission than U-235. In 1942, it was also discovered that U-238 could be transmuted into plutonium.
The pile
In 1942, in an indoor squash court in Chicago, Fermi and his team created what he called a “pile,” which was designed to create the first self-sustaining nuclear chain reaction. The pile was made of 50 tons of uranium combined with 360 tons of graphite. The graphite acted as a moderator, slowing the neutrons down to increase the chance of collision. Authorities were frightened that a chain reaction could cause serious damage in the densely populated city of Chicago, but Fermi convinced them that all necessary precautions had been taken. For safety, the pile contained four control rods that could be lifted into the pile to absorb neutrons and thereby regulate the reaction. Two control rods worked electrically, and a third one—just in case—was simply hung from a rope and could be cut with an axe in case the electricity malfunctioned. A fourth rod was slowly pulled out of the pile to get the chain reaction going. When this happened, the pile began to produce a steady amount of neutrons, indicating that the first man-made, self-sustaining nuclear reaction had become a reality.
Although the experiment showed that a nuclear reactor could work in principle, the pile only generated half a watt of power. Fermi hypothesized that further improvements to the pile might turn it into a useful energy source, but due to its weight, he saw “little likelihood of an atomic bomb, little proof that we were not pursuing a chimera.”
Fig. 628 – Drawing of the Fermi and his team working on the pile (U.S. National Archies and Records Administration)
The creation of the bomb
In the same year of Fermi’s experiment, the army took over the atomic bomb program, starting what became known as the Manhattan Project under the leadership of Leslie Groves (1896–1970). The United States invested a total of two billion dollars into the project. To keep it a secret, its facilities were built in remote locations and many of the workers involved had no idea they were working on a bomb.
One major production site for the bomb was Oak Ridge, which reached a peak population of about 40,000 workers and consumed about 15% of all the energy produced in the United States. On this terrain, the enormous Y-12 plant was used to separate U-235 from U-238. Despite a host of problems, it eventually produced about 200 grams of 12% enriched U-235. Oak Ridge also housed the X-10 facility, which contained a pile that produced a small amount of plutonium.
The small amounts of uranium and plutonium were sent to the research center at Los Alamos for further investigation. The Los Alamos team was headed by Robert Oppenheimer (1904–1967), who managed to convince many top scientists in the country to join the organization. The Los Alamos team discovered a method to detonate the bomb. The bomb required two chunks of enriched uranium that were on the verge of having the critical mass to sustain a chain reaction. These masses then had to be pressed together to form one mass in which the critical mass was exceeded, starting the reaction. The mechanism developed to do this was called the gadget. The plutonium bomb worked somewhat differently. In it, one mass of plutonium was compressed by explosives to start the reaction.
In 1944, two bombs were developed, one called Thin Man and the other called Fat Man, based on their shapes. When a detonation problem was discovered in Thin Man, it was later replaced by a lighter and smaller bomb known as Little Boy. In the same year, the plants at Oak Ridge began to produce enough uranium for the bombs.
Meanwhile, Colonel Paul Tibbets (1915–2007), the best bomber pilot in the Air Force, was ordered to run test flights, dropping 5500-pound orange dummy bombs. Since Germany was about to be defeated, the focus turned to Japan, where Tibbets began to fly to familiarize himself with the target area. Meanwhile, he was told nothing about the bomb. His commanding officer said:
We don’t know anything about it yet. We don’t know what it can do. […] You’ve got to make it to the airplane and determine the tactics, the training, and the ballistics—everything. These are all parts of your problem. This thing is going to be very big. I believe it has the potential and possibility of ending the war. [411]
Fig. 629 – Oppenheimer (c. 1944) (U.S. Federal Government)
Fig. 630 – Inside the Y-12 facility (U.S. Federal Government)
Fig. 631 – Fat Man (U.S. Federal Government)
The German pile
While the invasion of Germany was already underway, it was still unclear whether the Germans had been able to develop a bomb of their own. To investigate the matter, Groves sent a team to Europe, headed by Boris Pash (1900–1995). Pash eventually discovered research notes about the bomb in a German physics laboratory:
We studied the papers by candlelight for two days and nights until our eyes began to hurt [...] The conclusion was unmistakable. The evidence had proved definitely that Germany had no atom bomb and was not likely to have one in any reasonable form. [412]
Pash then set out to search for German atomic scientists. He finally found Werner Heisenberg (1901–1976), Otto Hahn, and various other scientists in a town called Haigerloch. In a research facility in the same town, the team discovered what turned out to be an atomic pile, but not one capable of sustaining a chain reaction.
The Trinity Test
Meanwhile, the Americans tested a plutonium bomb on a deserted spot close to Los Alamos under codename Trinity. The bomb was lifted to a tower and detonated, and scientists observed the explosion from a concrete bunker. The test was extremely risky as it was uncertain if the explosion would cause radioactive material to be shot into the upper atmosphere and spread over an enormous area. As a result, the army stood ready to evacuate the people in the surrounding area if necessary.
Fig. 632 – The bomb is raised to the top of Trinity Tower (U.S. Federal Government)
Even more worrying was the uncertainty as to whether the chain reaction in the bomb would ever stop. Groves wrote:
I had become a bit annoyed with Fermi […] when he suddenly offered to take wagers from his fellow scientists on whether or not the bomb would ignite the atmosphere, and if so, whether it would merely destroy New Mexico or destroy the world. He had also said that after all it wouldn’t make any difference whether the bomb went off or not because it would still have been a well worth-while scientific experiment. For if it did fail to go off, we would have proved that an atomic explosion was not possible. [412]
Some scientists, among them Edward Teller (1908–2003), positioned themselves 20 miles from the site. He recalled:
We were told to lie down on the sand, turn our faces away from the blast and bury our heads in our arms. No one complied. We were determined to look the beast in the eye. [412]
Instead, he applied sunscreen and looked at the explosion through thick welder’s glass.
Fig. 633 – The Trinity Test (U.S. Federal Government)
When the explosives inside the bomb were detonated, the plutonium sphere on the inside was compressed enough for it to reach critical density, starting a chain reaction that generated a temperature of millions of degrees and a pressure of millions of pounds—equal to the power of 15,000 tons of TNT. The explosion vaporized the tower and sent out a shockwave strong enough to tip over a container weighing more than 200 tons at a distance of half a mile from the explosion. It also knocked some of the observers to the ground, and some experienced temporary blindness from the bright light, despite looking through welder’s glass. After the blast, the characteristic mushroom cloud formed (see Fig. 633). The Atomic Age had begun.
Oppenheimer described his experience as follows:
We waited until the blast had passed, walked out of the shelter, and then it was extremely solemn. A few people laughed, a few people cried. I remembered the line from the Hindu scripture the Bhagavad-Gita: Vishnu is trying to persuade the prince that he could do his duty and to impress him takes on his multi-armed form and says, “Now I am become Death, the destroyers of worlds.” I suppose we all thought that, one way or another. [413]
Seeing the otherworldly destructive power of the bomb with their own eyes, it finally dawned on many of the scientists what a horrifying device they had created. Many began to worry about the unforeseen consequences of the bomb, especially if it would end up in the wrong hands. This fear would stay with them for the rest of their lives.
Hiroshima and Nagasaki
When Roosevelt died a few weeks before the surrender of Germany, Vice President Harry Truman (1884–1973) took over his office. It turned out even Truman had not been informed about the bomb. After he was updated about the Trinity test, he was told that a decision had to be made as to whether or not the bomb should be used against Japan. The debate about whether they made the right decision continues to this day. Some pointed to the fact that the Japanese would fight to the bitter end, which meant America had to instigate a full-fledged invasion of the country, leading to hundreds of thousands of deaths on American side. This, they reasoned, was too high a price to pay. Others, however, believed that the defeat of Japan was only a matter of time and preferred a naval blockade combined with conventional bombings.
Two months after Germany’s surrender, Truman met with Churchill and Stalin in Potsdam, Berlin, to discuss the ongoing war against Japan. When Truman informed the two about the Trinity test, Churchill was delighted, but Stalin showed little interest. Truman recalled:
I casually mentioned to Stalin that we had a new weapon of unusual destructive force. The Russian premier showed no special interest. All he said was that he was glad to hear it and hoped we would make “good use of it against the Japanese.” [414]
It later turned out that Russia already knew about the bomb as they had several informants working at the Manhattan Project.
The three leaders decided on the Potsdam Proclamation, which demanded Japan’s immediate surrender:
We call upon the government of Japan to proclaim now the unconditional surrender of all Japanese armed forces, and to provide proper and adequate assurances of their good faith in such action. The alternative for Japan is prompt and utter destruction. [415]
In the last sentence, the bomb was alluded to, but it wasn’t mentioned directly. This was because the Allied forces did not want to increase the chance of Japan shooting down the plane carrying the bomb. They were also worried that knowledge of the bomb might lead the Japanese to concentrate American prisoners of war in their cities as a deterrent. However, without mentioning the bomb, it gave the Japanese little reason to surrender. Japan still had an army of approximately two million men, and about 5000 airplanes and 3000 boats were packed with explosives for suicide attacks known as kamikaze.
As Japan refused to surrender, Tibbets received the order to drop Little Boy on Hiroshima. Fearing the 4400 kg bomb would crash on take-off, the bomb was made operational in the air. Once it was dropped, it released an energy equivalent to 15,000 tons of TNT, completely destroying about five miles of the city and vaporizing everything within a two-mile radius (see Fig. 634).
Fig. 634 – Hiroshima before and after the bomb (Japan)
Although the plane was already miles away when the bomb exploded, it was still rocked by the blast, making Tibbets believe he was taking flak. Looking back at the city, he recalled:
The city was hidden by that awful cloud, boiling up, mushrooming, terrible and incredibly tall. [416]
The tail gunner, Robert Caron, said:
My God, what have we done? [416]
Later Caron described the deadly mushroom cloud he saw forming behind him:
It’s like a mass of bubbling molasses. The mushroom is spreading out. It’s maybe a mile or two wide and half a mile high. It’s growing up and up. It’s nearly level with us and climbing. It’s very black but there is a purplish tint to the cloud. The base of the mushroom looks like a heavy undercast that is shot through with flames. [411]
Fig. 635 – Little Boy explodes over Hiroshima.
Fig. 636 – Fat Man over Nagasaki (1945) (U.S. Federal Government)
Fig. 637 – Hiroshima after the bomb (Japan)
It is estimated that about 100,000 people died because of the acute effects of the atomic bomb. More than 20,000 were schoolchildren. Many victims who survived the initial bombing died later of burns, radiation sickness, thyroid cancer, and leukemia. The men who survived exhibited a catastrophically low sperm count, and women often miscarried. Five years later, the total death count reached 200,000.
One survivor, a fourteen-year-old boy called Akihiro Takahashi, later told the story of what had happened to him:
We saw a B-29 approaching and about to fly over us. All of us were looking up at the sky, pointing out the aircraft. Then the teachers came out from the school building and the class leaders gave the command to fall in. Our faces were all shifted from the direction of the sky to that of the platform. That was the moment when the blast came. And then the tremendous noise came and we were left in the dark. I couldn’t see anything at the moment of explosion [...] We had been blown by the blast. Of course, I couldn’t realize this until the darkness disappeared. I was actually blown about 10 meters. My friends were all marked down on the ground by the blast. Everything collapsed for as far as I could see. I felt the city of Hiroshima had disappeared all of a sudden. Then I looked at myself and found my clothes had turned into rags due to the heat.
I was probably burned at the back of the head, on my back, on both arms and both legs. My skin was peeling and hanging. Automatically I began to walk, heading west because that was the direction of my home. After a while, I noticed somebody calling my name. I looked around and found a friend of mine who lived in my town and was studying at the same school. His name was Yamamoto. He was badly burnt just like myself.
We walked towards the river and on the way we saw many victims. I saw a man whose skin was completely peeled off the upper half of his body and a woman whose eyeballs were sticking out. Her whole body was bleeding. A mother and her baby were lying with their skin completely peeled off. We desperately made our way crawling. And finally, we reached the riverbank. At the same moment, a fire broke out. We made a narrow escape from the fire. If we had been slower by even one second, we would have been killed by the fire.
Fire was blowing into the sky, becoming four or even five meters high. There was a small wooden bridge left, which had not been destroyed by the blast. I went over to the other side of the river using that bridge. But Yamamoto was not with me anymore. He was lost somewhere. I remember I crossed the river by myself and on the other side, I plunged myself into the water three times. The heat was tremendous. And I felt like my body was burning all over. For my burning body the cold water of the river was as precious as a treasure.
Then I left the river, and I walked along the railroad tracks in the direction of my home. On the way, I ran into another friend of mine, Tokujiro Hatta. I wondered why the soles of his feet were badly burnt. It was unthinkable to get burned there. But it was undeniable fact the soles were peeling and red muscle was exposed. Even though I myself was terribly burnt, I could not go home ignoring him. I made him crawl using his arms and knees. Next, I made him stand on his heels and I supported him. We walked heading toward my home repeating the two methods. When we were resting because we were so exhausted, I found my grandfather’s brother and his wife, in other words, great uncle and great aunt, coming toward us. That was quite coincidence. As you know, we have a proverb about meeting Buddha in Hell. My encounter with my relatives at that time was just like that. They seem to be the Buddha to me wandering in the living hell. [417]
Within a few hours, President Truman informed the American public about the bomb with a prepared radio statement. Meanwhile, the Japanese authorities lied to the survivors of Hiroshima, claiming that Japan had retaliated using “the same mysterious weapon” against major cities in California. To the rest of the country, however, they remained silent about the bomb. [409]
When the Japanese still refused to surrender, the Americans ordered a second attack, which most historians agree now believe was ethically wrong, even if it meant ending the Second World War. This time, they released Fat Man on Nagasaki, which housed large Mitsubishi factories involved in war production. With no one informed about the first bomb, the people in Nagasaki were just as unprepared as the people in Hiroshima had been.
After the second bomb, Emperor Hirohito (1901–1989) was finally willing to surrender, believing that “to continue the war meant nothing but the destruction of the whole nation.” “The time has come,” he told his government, “when we must endure the unendurable.” [409] The next day, he broadcasted a message, calling for all Japanese to accept the surrender. He warned them to “beware of any outburst of emotion,” as many Japanese were ready to fight to the death. As one admiral recounted, “even when [...] the imperial decree to terminate the war was actually issued, we found it difficult to hold down the front-line forces who were all ready to go.” [409] Many committed suicide, unable to bear the defeat. A small group of officers in the palace of the emperor even attempted a coup to keep the war going. They tried to put the emperor under house arrest and destroy the recording of the emperor’s surrender before it was broadcasted. When their coup failed, the responsible officers committed hara-kiri (suicide by slicing open the abdomen with a knife).
On September 2, 1945, the Japanese foreign minister signed the surrender document on board the USS Missouri in Tokyo Bay. The Second World War was finally over.
Doubts about the bomb
Looking back on the Manhattan Project, many of the contributing scientists had mixed feelings. Oppenheimer wrote:
I have no remorse about the making of the bomb and Trinity. That was done right. As for how we used it, I understand why it happened and appreciate with what nobility those men with whom I’d worked made their decision. But I do not have the feeling that it was done right. The ultimatum to Japan was full of pious platitudes. [...] Our government should have acted with more foresight and clarity in telling the world and Japan what the bomb meant.
Oppenheimer was clearly aware of the dangers of his invention:
If atomic bombs are to be added as new weapons to the arsenals of a warring world, or to the arsenals of the nations preparing for war, then the time will come when mankind will curse the names of Los Alamos and Hiroshima. The people of this world must unite or they will perish. [418]
Einstein also had many worries. In 1954, five months before his death, he summarized his feelings about his role in the creation of the atomic bomb:
I made one great mistake in my life, [...] when I signed the letter to President Roosevelt recommending that atom bombs be made; but there was some justification—the danger that the Germans would make them. [419]
On another occasion, he said:
If I had known that the Germans would not succeed in constructing the bomb, I would never have lifted a finger. [411]
The hydrogen bomb
In 1946, the Manhattan Project tested two more bombs, the second of which produced a spectacular display for the world to see (see Fig. 638).
In 1949, the Soviet Union caught up and developed its own atomic bomb, further heightening the tension of the Cold War. In the arms race that followed, both countries produced a nuclear arsenal large enough to destroy the world many times over.
In 1952, the United States tested its first hydrogen bomb, Ivy Mike, which was even more destructive. In 1954, the first lithium-deuteride bomb, Castle Bravo, was detonated (see Fig. 639). Its yield was 15 megatons of TNT, and it led to unexpected radioactive contamination of the area, causing the inhabitants to suffer from radiation sickness. Radioactive fallout from the explosion spread through the atmosphere around the world.
Fig. 638 – The Bikini Atoll nuclear test of 1946 (U.S. Federal Government)
Fig. 639 – Castle Bravo nuclear test (1954) (U.S. Federal Government)
The Cuban Missile Crisis of 1962 was the closest the world has ever come to full-scale nuclear war. In 1962, the Americans discovered that Khrushchev had made agreements with Fidel Castro to deploy ballistic nuclear missiles in Cuba to deter an American invasion. After several days of negotiations, the tension got too high for both parties. Russia agreed to publicly dismantle their weapons. In exchange, the United States publicly declared it would not invade Cuba.
Epilogue
And there we have it, our history from our primal beginnings to modern times. We have witnessed the building of civilizations and the development of the great intellectual, artistic, and religious traditions. We have ventured eastward to India, China, and Japan, where an exploration of the mind helped people see reality unfiltered. Then we ventured westward, where we witnessed the formulation of logic and mathematics in Greece and the development of the scientific method in Western Europe. Emblematic for the West was also its ever-increasing concern and care for the individual. This development had its clearest expression in law, where human rights were formulated to protect the individual against injustice.
Our story ended rather bleakly with the violent excesses of the Soviet Union and Nazi Germany and the destructive power of the atomic bomb. However, there is also cause for optimism. Over the last century, liberal democracies, with their rule of law, representative democracy, protection of human rights, and successful market economies, have been on the rise (albeit with some ups and downs). At the same time, war and poverty have been on the decline worldwide.
There is also much excitement about new developments in the world. In recent decades, the world has entered the Digital Age with the creation of computers and mobile devices. Starting in the 50s, we have also entered the Space Age, its most monumental moment still being humankind’s first step on the moon in 1969.
But what adventures (or horrors) lie ahead? Only time will tell. What seems clear, is that we must cherish the lessons learned by our ancestors, who—as the Irish writer James Joyce (1882–1941) phrased it—have “forged in the smithy of [their] soul the uncreated conscience of [our] race.”
Fig. 640 – Earthrise taken from the Apollo 8 in orbit around the moon by Bill Anders (1968) (Nasa)